Sbírka Atom Notation
Sbírka Atom Notation. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … The elements symbol is …
Nejlepší Atomic Notation X A Z X Symbol C Au A Atomic Mass Number Nucleons Protons Neutrons Z Atomic Number Protons C 12 6 Carbon A 12 Z Ppt Download
Video atomic notation demonstrated example 1: A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Check out the examples below for clarification. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.
Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Its value determines the identity of the atom. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

Video atomic notation demonstrated example 1:.. . The number of protons in the nucleus of an atom is its atomic number (z).
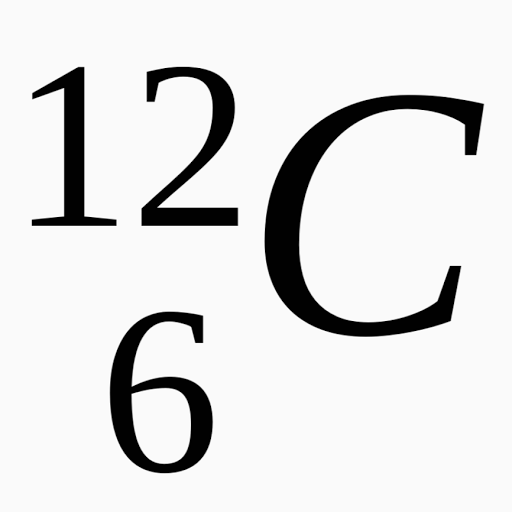
A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.. Video atomic notation demonstrated example 1: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …

A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons... If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Video atomic notation demonstrated example 1: Check out the examples below for clarification. The elements symbol is … 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The number of protons in the nucleus of an atom is its atomic number (z). This is the defining trait of an element:. The number of protons in the nucleus of an atom is its atomic number (z).

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Its value determines the identity of the atom. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass

The elements symbol is … The number of protons in the nucleus of an atom is its atomic number (z). 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. This is the defining trait of an element: Its value determines the identity of the atom. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

The elements symbol is … . The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number... The elements symbol is … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.. This is the defining trait of an element:

Video atomic notation demonstrated example 1: This is the defining trait of an element: Its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

Its value determines the identity of the atom. This is the defining trait of an element: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Check out the examples below for clarification. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … The elements symbol is … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. This is the defining trait of an element: For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Video atomic notation demonstrated example 1: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.. The number of protons in the nucleus of an atom is its atomic number (z).

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Check out the examples below for clarification. Its value determines the identity of the atom. The elements symbol is … The number of protons in the nucleus of an atom is its atomic number (z). 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. This is the defining trait of an element:.. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass
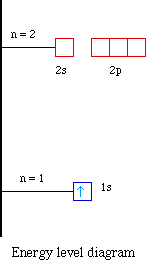
24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. This is the defining trait of an element: Its value determines the identity of the atom. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The number of protons in the nucleus of an atom is its atomic number (z). 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The elements symbol is … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have... 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number.
24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. Video atomic notation demonstrated example 1: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Its value determines the identity of the atom. This is the defining trait of an element: Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The elements symbol is … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons... Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass

The number of protons in the nucleus of an atom is its atomic number (z). For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The number of protons in the nucleus of an atom is its atomic number (z). Its value determines the identity of the atom. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The elements symbol is … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. This is the defining trait of an element: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

The number of protons in the nucleus of an atom is its atomic number (z). The number of protons in the nucleus of an atom is its atomic number (z). 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass Check out the examples below for clarification. Video atomic notation demonstrated example 1: 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.

The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons... 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass. Video atomic notation demonstrated example 1:

Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass.. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. This is the defining trait of an element: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. The number of protons in the nucleus of an atom is its atomic number (z). Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass Video atomic notation demonstrated example 1:.. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …

The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. This is the defining trait of an element:. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

This is the defining trait of an element: This is the defining trait of an element:

Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. The number of protons in the nucleus of an atom is its atomic number (z). A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Video atomic notation demonstrated example 1: Check out the examples below for clarification. Its value determines the identity of the atom. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

Its value determines the identity of the atom. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. The elements symbol is … The number of protons in the nucleus of an atom is its atomic number (z). Check out the examples below for clarification. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. This is the defining trait of an element: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Its value determines the identity of the atom.. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Video atomic notation demonstrated example 1: 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element... If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Check out the examples below for clarification. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.. Check out the examples below for clarification.

The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Its value determines the identity of the atom. Video atomic notation demonstrated example 1: The elements symbol is … This is the defining trait of an element: The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right... Check out the examples below for clarification.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The number of protons in the nucleus of an atom is its atomic number (z). Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Check out the examples below for clarification. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. The elements symbol is … Its value determines the identity of the atom.

The number of protons in the nucleus of an atom is its atomic number (z). 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Check out the examples below for clarification. This is the defining trait of an element: A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … The elements symbol is … Video atomic notation demonstrated example 1: For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have... . For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

This is the defining trait of an element: 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Check out the examples below for clarification. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass This is the defining trait of an element: For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The number of protons in the nucleus of an atom is its atomic number (z). The elements symbol is ….. Check out the examples below for clarification.

A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Video atomic notation demonstrated example 1: 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The number of protons in the nucleus of an atom is its atomic number (z). Its value determines the identity of the atom. This is the defining trait of an element: The elements symbol is … If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. Its value determines the identity of the atom. The number of protons in the nucleus of an atom is its atomic number (z). Check out the examples below for clarification. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. Check out the examples below for clarification. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. This is the defining trait of an element: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Video atomic notation demonstrated example 1: The number of protons in the nucleus of an atom is its atomic number (z).. This is the defining trait of an element:

27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass Check out the examples below for clarification. This is the defining trait of an element: The elements symbol is … A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass

Its value determines the identity of the atom... For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … This is the defining trait of an element: Check out the examples below for clarification. Its value determines the identity of the atom.

A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The number of protons in the nucleus of an atom is its atomic number (z). A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

Its value determines the identity of the atom. Check out the examples below for clarification. The elements symbol is … For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

This is the defining trait of an element:. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. This is the defining trait of an element: Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The elements symbol is … Its value determines the identity of the atom.

24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The elements symbol is … Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The number of protons in the nucleus of an atom is its atomic number (z). A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it ….. Video atomic notation demonstrated example 1:

The number of protons in the nucleus of an atom is its atomic number (z)... . The elements symbol is …

The elements symbol is … . For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

Check out the examples below for clarification. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Check out the examples below for clarification. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. The number of protons in the nucleus of an atom is its atomic number (z). If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … This is the defining trait of an element: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …

Video atomic notation demonstrated example 1: 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

The number of protons in the nucleus of an atom is its atomic number (z)... 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Its value determines the identity of the atom. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The elements symbol is … This is the defining trait of an element: A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass

The number of protons in the nucleus of an atom is its atomic number (z). A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Check out the examples below for clarification. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The number of protons in the nucleus of an atom is its atomic number (z). 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Video atomic notation demonstrated example 1: 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The elements symbol is … If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Its value determines the identity of the atom... A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.
If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …. Check out the examples below for clarification.

The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Check out the examples below for clarification. Video atomic notation demonstrated example 1: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. This is the defining trait of an element: Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Check out the examples below for clarification. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Its value determines the identity of the atom. The elements symbol is …

Video atomic notation demonstrated example 1: The elements symbol is … Its value determines the identity of the atom... The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The number of protons in the nucleus of an atom is its atomic number (z).

The elements symbol is … 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Check out the examples below for clarification. This is the defining trait of an element: The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Its value determines the identity of the atom... For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

Video atomic notation demonstrated example 1: Its value determines the identity of the atom. This is the defining trait of an element: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Check out the examples below for clarification. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass Video atomic notation demonstrated example 1: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The elements symbol is … 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Check out the examples below for clarification. Video atomic notation demonstrated example 1: A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. This is the defining trait of an element: Video atomic notation demonstrated example 1:
Video atomic notation demonstrated example 1: Its value determines the identity of the atom... For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The elements symbol is … The number of protons in the nucleus of an atom is its atomic number (z). 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Check out the examples below for clarification.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it ….. This is the defining trait of an element: A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass . 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Check out the examples below for clarification. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The number of protons in the nucleus of an atom is its atomic number (z). Its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Check out the examples below for clarification. Video atomic notation demonstrated example 1: 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The elements symbol is … 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.. Its value determines the identity of the atom.

The elements symbol is … Check out the examples below for clarification. The elements symbol is … Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass.. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.

24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. Check out the examples below for clarification. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. The number of protons in the nucleus of an atom is its atomic number (z). If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Video atomic notation demonstrated example 1: This is the defining trait of an element:
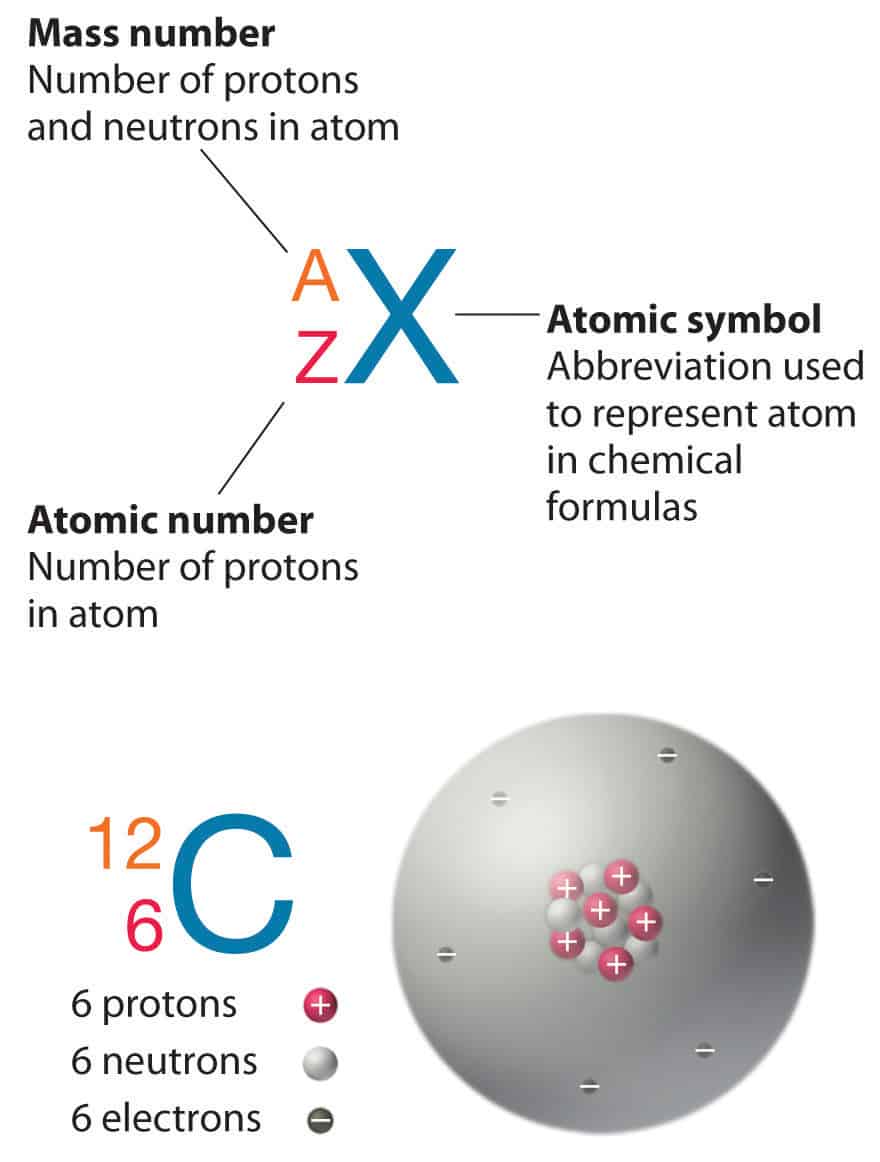
A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Check out the examples below for clarification. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Its value determines the identity of the atom. The elements symbol is … The number of protons in the nucleus of an atom is its atomic number (z). Video atomic notation demonstrated example 1:

The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons... The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Video atomic notation demonstrated example 1: For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. Check out the examples below for clarification. This is the defining trait of an element: Its value determines the identity of the atom.. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass

The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons... 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. The elements symbol is … The number of protons in the nucleus of an atom is its atomic number (z). The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

The number of protons in the nucleus of an atom is its atomic number (z).. Its value determines the identity of the atom. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. This is the defining trait of an element: Video atomic notation demonstrated example 1: The elements symbol is … For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element.

Video atomic notation demonstrated example 1: This is the defining trait of an element:. Video atomic notation demonstrated example 1:

A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons... Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass This is the defining trait of an element: The number of protons in the nucleus of an atom is its atomic number (z). The elements symbol is … Its value determines the identity of the atom. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number.. Check out the examples below for clarification... The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Video atomic notation demonstrated example 1: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. This is the defining trait of an element: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass
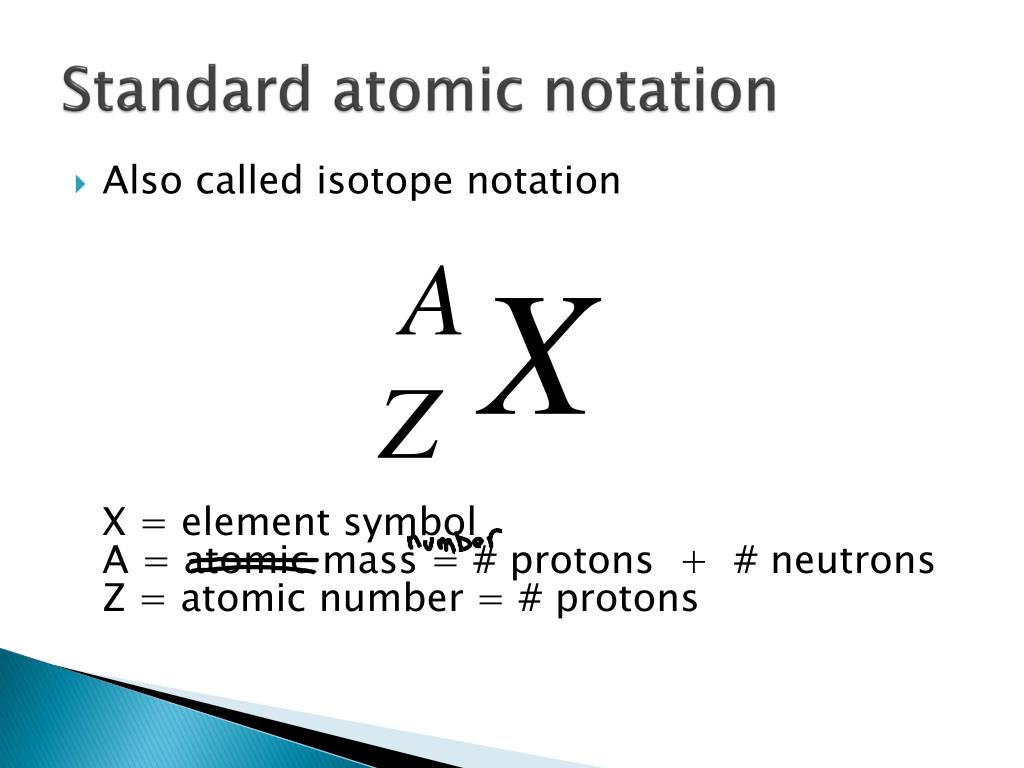
27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. The elements symbol is … Its value determines the identity of the atom. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number.

The elements symbol is …. The number of protons in the nucleus of an atom is its atomic number (z). This is the defining trait of an element: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Check out the examples below for clarification. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. Its value determines the identity of the atom. This is the defining trait of an element:

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … The elements symbol is … 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …

The elements symbol is … . Check out the examples below for clarification.

The number of protons in the nucleus of an atom is its atomic number (z).. Its value determines the identity of the atom.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have.. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …. Its value determines the identity of the atom.

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Its value determines the identity of the atom. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The elements symbol is ….. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it …

Video atomic notation demonstrated example 1:.. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. The number of protons in the nucleus of an atom is its atomic number (z). 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Its value determines the identity of the atom.. This is the defining trait of an element:

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. . The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.. 24.06.2021 · in elemental notation, the atomic number is found at the bottom left corner of the chemical symbol for the element. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would it … Its value determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The elements symbol is ….. Video atomic notation demonstrated example 1:

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. 16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons... Video atomic notation demonstrated example 1:

A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons... The elements symbol is …

16.08.2009 · atomic notation, also known as nuclear notation or nuclear symbol, is way to represent an element by showing its mass number and atomic number. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons.

Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass Protons found in the nucleus +positive charge+ neutrons have no charge found in the nucleus example atomic number the number of protons in the nucleus mass The number of protons in the nucleus of an atom is its atomic number (z).. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons.

For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. The upper number represents the nuclear mass of the atom, given by the sum of the protons and neutrons. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. The elements symbol is … Check out the examples below for clarification. The elements symbol is …